Smart IV Cannulation Assist Device
Real-time, AI-driven vein imaging for safer and effortless cannulation.
A next-generation tabletop device that enhances vein visibility using optimized illumination, real-time image processing, and ML-based detection—designed to support accurate IV access with minimal human intervention.
Our Key Features
Our Smart IV Cannulation Assist Device combines optimized illumination, real-time image processing, and AI-based vein detection to deliver highly accurate and reliable vascular imaging. Designed for hands-free operation with an intuitive HMI, it enhances clinical efficiency and improves cannulation success rates.
Customized Illumination
Real-Time Image Processing
ML-Based Vein Detection & Segmentation
Hands-Free Operation
Intuitive HMI
Portable Tabletop Design
How It Works
01.
Positioning
User places hand or forearm on the imaging platform
02.
Illumination & Capture
Device illuminates the tissue; camera acquires raw image data
03.
Real-Time Enhancement
Algorithms enhance contrast and highlight vein structures
04.
AI Segmentation
Machine-learning model maps veins, bifurcations, and insertion points
05.
Visual Guidance
HMI displays colored overlays, distances, and access recommendations
06.
Logging & Export
Images and metadata can be saved or exported for clinical records


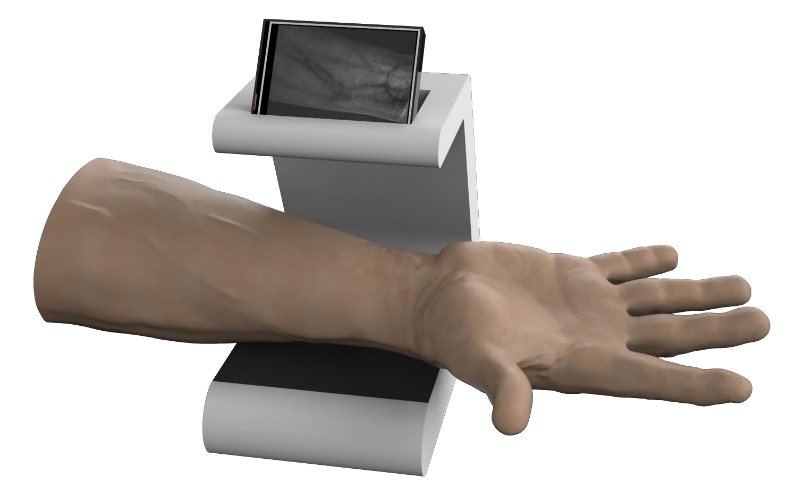





The images showcase the Smart IV Cannulation Assist Device’s real prototype, including its tabletop structure, camera and NIR illumination module, HMI display, and arm placement platform. They visually demonstrate both the raw and processed vein images, highlighting real-time vein enhancement and the device’s functional design.
Why Choose Macvins for Smart IV Cannulation Assist Device
Macvins delivers reliable, high-precision biomedical solutions backed by deep technical expertise, rigorous testing, and end-to-end support—trusted by hospitals, researchers, and healthcare innovators.
We combine biomedical engineering, AI expertise, and clinical insight to develop high-precision healthcare solutions that improve patient outcomes and workflow efficiency.
Every device undergoes rigorous research, prototyping, testing, and validation—including bench tests, phantom simulations, and clinical evaluations—to ensure consistent performance and safety.
Yes. From development and customization to deployment, training, and post-installation assistance, we offer complete end-to-end support for seamless integration into clinical settings.
Our development process follows global medical standards such as ISO 14971, IEC 62304, and IEC 60601, ensuring safety, reliability, and regulatory compliance.
Hospitals, research institutions, universities, and healthcare startups rely on our expertise and innovation to enhance diagnostics, treatment precision, and clinical efficiency.
